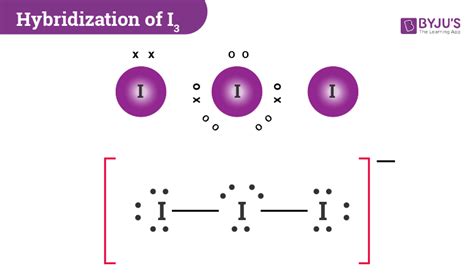
i3- hybridization|i3 hybridization of central atom : Baguio Learn how to draw the Lewis structure of I3- or triiodide ion, a polyatomic ion with 22 valence electrons and sp3d hybridization. Find . Generate free keyword ideas for Amazon in 171 countries, complete with monthly search volumes. . Enter a broad description of your product into Ahrefs' Amazon Keyword Tool and check one of the five keyword ideas reports .
i3- hybridization,Learn how to calculate the hybridization of I3- ion, a linear anion formed by the bonding of I2 with I- ion. Find out the number of valence electrons, lone pairs, bond angles and molecular geometry of I3-. Tingnan ang higit pa
i3- hybridizationTo know the hybridizationof Triiodide ion, we can use simple hybridization formula which is given as; If we look at the iodine atoms there are seven valence electrons in its outer shell and two monovalent atoms are also present. Further, during the combination . Tingnan ang higit pa

I3- molecular geometry is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives 3 . Tingnan ang higit pa
Learn how to draw the Lewis structure of I3- or triiodide ion, a polyatomic ion with 22 valence electrons and sp3d hybridization. Find . Learn how to draw the Lewis structure of I3- ion, a polyatomic molecule with a negative charge, and how to calculate its hybridization, molecular geometry, and polarity. Find out the properties and uses of I3- . I3⁻ exhibits a linear geometry with bond angles of 180°, consistent with sp³d hybridization. The presence of the extra electron on the central iodine contributes to the . Learn how to determine the hybridization of I3-, a linear anion with sp3d hybridization, by using the formula, lone pairs and valence electrons. See the Lewis . This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges of .
An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii.
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.
I 3- is dsp 3 hybridized and contains 3 lone pairs and 2 bonding pairs of valence electrons around the Iodine. The VSEPR predicts the linear shape. Elements in the first 2 periods . First things first: the bonding orbitals of $\ce{I3-}$ do not contain any significant d-orbital contributions. In fact, the $\ce{I-I}$ bond lengths are significantly longer than in $\ce{I2}$ , suggesting a lower .Triiodide is a model system in photochemistry. Its reaction mechanism has been studied in gas phase, solution and the solid state. In gas phase, the reaction proceeds in multiple pathways that include iodine molecule, metastable ions and iodine radicals as photoproducts, which are formed by two-body and three-body dissociation.HYBRIDIZATION OF I3. To know the crossbreeding of Triiodide particle, we will use a straightforward crossbreeding formula that is given as; Number of crossbreeding = electron + monovalent + (negative charge) – . This indicates that the hybridization of I3- is sp 3 d. Another way to determine the hybridization of I3- is by counting the number of valence electrons and lone pairs and adding them together. In this case, we have 3 lone pairs and 2 atoms donating valence electrons, giving us a total of 5, which also indicates sp 3 d hybridization. Key . Orbital hybridization The observation of molecules in the various electronic shapes shown above is, at first blush, in conflict with our picture of atomic orbitals. For an atom such as oxygen, we know that the 2s orbital is spherical, and that the 2p x , 2p y , and 2p z orbitals are dumbell-shaped and point along the Cartesian axes. #Hybridization of (I)3- , CHEMICAL BONDING#I3-,(I)3-#studyBibhaschemical bonding class 11,chemical bonding video lecture,hybridisation class 11 iit jee,how t.
sp³ hybridization. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of . I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is .Click here:point_up_2:to get an answer to your question :writing_hand:the hybridisation of central iodine atom in if5 i3 and i3 are respectivelysp Hybridization. The beryllium atom in a gaseous BeCl 2 molecule is an example of a central atom with no lone pairs of electrons in a linear arrangement of three atoms. There are two regions of valence electron density in the BeCl 2 molecule that correspond to the two covalent Be–Cl bonds. To accommodate these two electron domains, two of the Be .
Solution. The correct option is D Both are planar species. Here the hybridisation of central atom in I + 3 is sp3 and it contains 2 lone pairs on it. So, I + 3 becomes bent shaped, planar and is polar. The hybridisation of central atom in I − 3 is sp3d and it contains 3 lone pairs on it. The lone pairs are present on equatorial axis and two . I3- ion has sp3d hybridization and linear shape containing two bond pair and three lone pair while I3+ ion has sp3 hybridization and bent shape containing two bond pair and two lone pair. Related .i3 hybridization of central atom Triiodide [I3]- ion Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polar vs non-polar. The chemical formula I 3– represents an anion composed .The hybridization of an atom is determined based on the number of regions of electron density that surround it. The geometrical arrangements characteristic of the various sets of hybrid orbitals are shown in Figure 8.21. These arrangements are identical to those of the electron-pair geometries predicted by VSEPR theory.

Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z . First things first: the bonding orbitals of $\ce{I3-}$ do not contain any significant d-orbital contributions.In fact, the $\ce{I-I}$ bond lengths are significantly longer than in $\ce{I2}$, suggesting a lower bond order. In fact, the triiodide anion should rather be imagined as a 4-electron-3-centre bond (bond order approximately 0.5) which might .
i3- hybridization i3 hybridization of central atomI3- is sp3d hybridized and contains 3 lone pairs and 2 bonding pairs of valence electrons around the Iodine. The VSEPR predicts the linear shape. Three of the sp3d orbitals are occupied by 3 lone pairs and the other two orbitals form bonds with the other Iodine atoms.'. Suggest Corrections. 1. This video illustrates a step-by-step process in deriving the electronic geometry, molecular geometry, hybridization of the central atom, and formal charge o.
i3- hybridization|i3 hybridization of central atom
PH0 · what is hybridization in chemistry
PH1 · what does sp3 hybridized mean
PH2 · sp hybridization chart
PH3 · lone pairs in i3
PH4 · i3 lewis structure molecular geometry
PH5 · i3 hybridization of central atom
PH6 · i3 electron domain geometry
PH7 · hybridization chemistry for dummies
PH8 · Iba pa